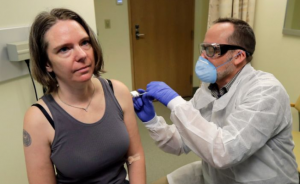
The first person in the world was given a dose of a potential coronavirus vaccine on Monday.
The start of a clinical trial marks historically fast progress in developing a vaccine in the face of a pandemic. The virus was genetically sequenced only about two months ago.
But the experimental vaccine has a long way to go to reach a broader population. The study that just started is primarily focused on making sure the vaccine is safe in humans. If it is, future trials will test larger groups of people to determine whether it can actually prevent infection.
Clinical testing will take at least a year to 18 months to determine whether the vaccine is safe and effective, Anthony Fauci, the longtime head of the National Institutes of Health’s infectious-disease unit, has repeatedly said.
Read Also:
Attacks on Christian cemeteries alarm Turkey’s Christians
Thousands of Afghans return from Iran as virus hits economy
If it were to be approved, it would be the first vaccine developed with a novel technology platform using messenger RNA. The technology was a critical component of the ability of the vaccine’s developer, a small Massachusetts company called Moderna, to move so quickly.
Ask me anything
Explore related questions