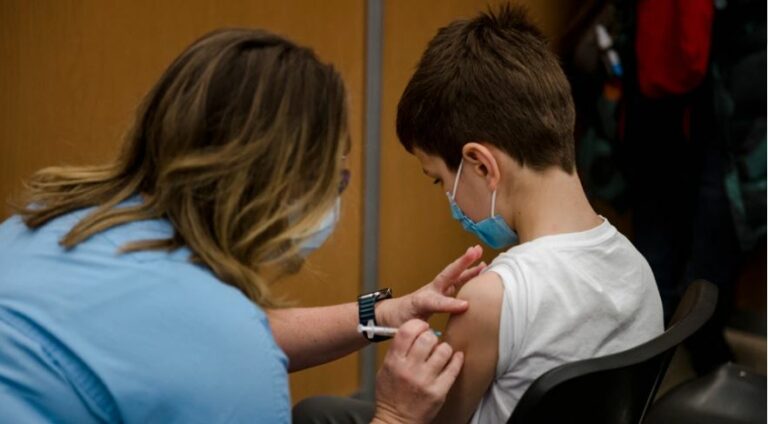The European Medicines Agency (EMA) Committee for Medicinal Products for Human Use (CHMP) today recommended that the Pfizer / BioNTech vaccine be given to children aged 5 to 11 years. To date, the vaccine has been approved for use in adults and children 12 years of age and older. Following the recommendations by EMA, European countries usually approve the vaccine.
In children aged 5 to 11 years, the dose of Comirnaty (as the German and American company vaccine is called) will be lower than that used in people 12 years of age and older (10 mg compared to 30 mg). As in the older age group, it is administered intramuscularly in two doses three weeks apart.
A major study in children aged 5 to 11 years showed that the immune response to Comirnaty given at a lower dose (10 mg) in this age group was comparable to that observed at the highest dose (30 mg) at 16 to 25 years of age. The efficacy of Comirnaty was estimated in nearly 2,000 children aged 5 to 11 years who had no signs of a previous infection. These children received either the vaccine or a placebo. Of the 1,305 children who received the vaccine, three developed COVID-19 compared with 16 of the 663 children who received a placebo. This means that, in this study, the vaccine was 90.7% effective in preventing symptomatic COVID-19 (although the actual rate could be between 67.7% and 98.3%).
Ask me anything
Explore related questions